This was originally published here, in French (link).
We provide this translation for your convenience. Practical aspects may differ where you live.
DTG-25 mg episode 4
By Charles-Edouard!Here's a Patrick who would have done better to understand intermittency than to play the fool.
This is what the omerta on the intermittence gives: the moral responsibility is in the opposite camp. Sensitive to false testimony, and with a mind obviously clouded by overmedication, this is one person who has been totally closed to our arguments. ARVs freeze the situation; in the absence of active replication, the lymphocytes return to a more usual equilibrium. When they thaw out, they return to their former equilibrium in a few months. Therapeutic vacancy in LOTTI or SALTO conditions is favorable. For those who have already passed through an AIDS stage, such as our Patrick, it is the announced disaster (some of them manage to control their disease for a fairly long period of time: the PTC).
The septists have the double punishment: the infamous responsibility of overmedication, and, boomerang effect, the aforementioned Patrick will finally understand that intermittence (ICCARRE/OMNIBVS) is the uninterrupted maintenance ofthe viremic suppression. Let's hear it...
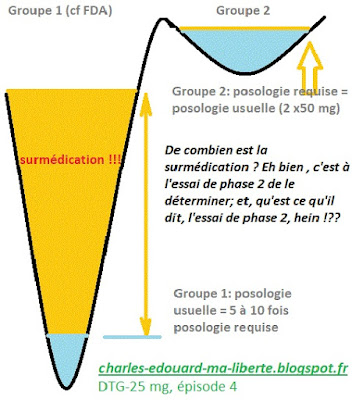
DTG-25 mg: necessary dosage vs usual dosage
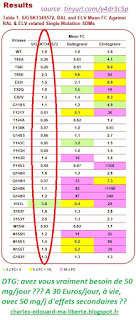
The two tables, made public by ViiV(see episode 3), are rich in information: there are no yellow, orange or red primary mutations under DTG, but there are red ones under RAL or EVG: under RAL or DTG, you almost inevitably need 2 other molecules to block the possible appearance of mutations that would make RAL or EVG inoperative. Under the old INIs, these red boxes require IRT, including in group 1 (naive or successful patients), or failing that, in maintenance, a BI in a well-synergistic couple. Moreover, EVG is ONLY available in IRT, and the admissible reason is this bright red table; without even mentioning the second table (successive mutations).
On the other hand, it is not surprising that MONO-DTG works! It is right there under your nose! And obviously, if you have been through RAL or EVG you are at risk: secondary mutations may have appeared surreptitiously, and you may be in the case of table 2, and there a third molecule (or a double dose of DTG) are useful or even necessary. No one will ever tell you that, Merck because RAL is on its last legs, Gilead because they hope to convert EVG to BTG (Biktarvy) and ViiV because Mono-DTG takes 25% of their profit. Not even the FDA, because their specification is flawed.
I made a small synthetic diagram, inspired by the poster of Seki (ViiV, CROI-2010). Here, it is very clear!
Phase 2 ??? What phase 2? Nothing to beat...
You, you thought blithely that the phase 2 trial is used to determine the minimum dose required for maximum effectivenessYou try several doses and you take the one that has the best result, at the lowest dosage. For Evafirenz, the trial is clear: 200 mg should have been chosen, and many suicides would have been avoided and many more patients would have had access to treatment. The Phase 2 trial serves a purpose, otherwise why do it? Well no... We do it and we don't care... The proof by Fujiwara(ViiV powerpoint, here):
Maximum tolerated dose is not minimum effective dose!... But the most shocking is the 'A priori...' which is defined as follows:
1. Starting from data prior to the experiment.
2. At first sight, before any experiment.
If you choose the dose BEFORE the experiment, you have made a decision, on paper, without any intention of taking the phase 2 trial into account. The trial is mandatory, but it is not mandatory to take it into account!!!
So what do you do if you can't tolerate the maximum tolerated dose (like 10-15% of patients, at 3 years)?(see ANRS study)? Do you switch? And for what? And why? Whether you tolerate it more or less well, or even very well, why stuff yourself at 30 Euros per day?
So you have those who laugh at the effectiveness of DTG, some who regret the dropout rate, higher than in the advertisement. But, not one to go and look at the phase 2 trial. What is the point of trusting doctors who don't read the trials. Did they get their diploma in a Bonux package? And, when did they get this old-fashioned sesame?
Scam? But where is the scam?
Indeed, someone takes a zirconia and sells it to you as a diamond, there is deception; if a 300 mg pill has only 100 mg (or plaster), there is deception. But how does the manufacturer benefit from overdosing? Try to think about it...
Let's take an example closer than rosuvastatin:
For HIV, Lamivudine is in daily dosage at 300 mg/d. at a price of 69.42 ���� (Reimbursement rate: 100%) by Mylan for 60 tablets of 150 mg, scored (source)
For HBV, the same Lamivudine is in a dosage of 100 mg/d. Price : 88,43 € Reimbursement rate : 65% for 30 tablets(source)
For 69 Euros, reimbursed, you have 60 pills of 150 mg; for 88 Euros, you have only 30 pills (100 mg): it is twice as expensive (taking into account the reimbursement), for 3 times less!
The average Frenchman doesn't see the trick, but the average American, without social security coverage, quickly understands that buying Mylan 300 mg (in fact 150mg x 2), cutting it in 2 (or in 2/3) will save him $50 per month. $600 a year for the little lamivudine of my two....
When the price is crushed, i.e. not in proportion to the dose, then the retired, the poor, the parents of infected children, all will jump on the bargain; and it is as much loss of profit for the manufacturer...
A little thought before the next step
To get a head start on what's next, try to imagine for yourself what a base dosage of 10 mg would have meant for the healthcare system, for profits, and also for yourself, as part of maintenance.
Some people think that by doing 1/2, they are doing intermittence, the only proven way to dynamic remission: this is incorrect: they are only correcting the dose, wisely, as we will see.
Others say that if they do 3/7 or 2/7, they are lucky to be the 'happy few', the privileged ones... But where do you get that from? Do you know the failure rate for 3/7 maintenance under Triumeq ® ? Have you ever seen someone fail at 3/7 (or even 2/7)? In MONO-DTG, if we are not careful, there are failures, but not in those who minimize the risk (good compliance, no Achilles' heel and/or early initiation). So there, Happy, Yes... Few, No!...
When injectables arrive, at an exorbitant price, are you really going to inject the whole dose? every month? For life???
If you think that overdosing is a malpractice, look to your doctor, who, in the chain, is the last but one before you. The doctor has every right to advise you to take half a Tivicay®. It's his job, the useless privilege of not using it
Over-medication is an opportunity, as long as you realize it and take advantage of it
OMNIBVS
I'm in OMNIBVS all the way and, despite a unheard of adversityI've just won a decisive move. A great thing that changes the game, so morale is up!
Especially since the first batch of 1/15 is on its way! This is also really important and as soon as I'm done with DTG-25 mg, I'll give you my thoughts about the transition from 2/7 (Triumeq® or Eviplera®/Odyfsey®) to 1/7, then 1/15. Yours truly continues his successful descent, currently at 1/10, with the NFC (Nouvelle Formule de Charles-Edouard). The first one is for those who will have provided an email address, which can be done simply by leaving a message here(email addresses are not published, of course).
Judiciarization
- We had announced here that ViiV filed a complaint against Gilead for plagiarism (Bictegravir). We will soon resume the investigation. Indeed, new, imperceptible movements give us the keys to a secret YALTA, which redefines, in all discretion, the new power relations in the medical-pharmaceutical underworld. To be continued...
In the news
- Massive attack on Nevirapine. The disappearance of Videx® from the shelves has taken the orthodox iccarrians by surprise. They were almost the last users in France. Eventually, Videx® will have to be replaced... This explains the urgency to launch OMNIBVS without delay. I don't understand why they don't see what's coming, confident as they are in their temporary solution: they are wrong. By the way, this is exactly why I didn't want to get on that train, even though it was well underway, and why no clinician wants to take up the torch from that angle. It's a shame, but hey... We'll find a solution... I mean... We'll... Other than me, I don't see a lot of people...
I have more confidence in Nevirapine, which will always be marketed in India or China... But in France? Its days are counted if we are not careful. I will comment further, get ahead: Nevirapine Explainer May 2018 (US Congress), the DTL strategy.
Good Weekend, good stuffing and not too many meds ... Right?






No comments:
New comments are not allowed.