This paper was originally published here, in French. We provide the google translation for your convenience. Proper translation will come soon. Some practical aspects may differ where you live.
The press release comes from here https://www.sciencedaily.com/releases/2016/07/160719091625.htm
Here is a direct link to the poster. A summary was presented by thebody.com but not impartially (owned by a commercial company under private law).
The poster also downloaded from the page: Complete file; it is recommended to download the ZIP, this is simpler. The poster is then in directory: dossier_complet\relief\ICCARRE\clinical trials\ANRS-162-4D
This what ANRS-4D uncovered
In short:
- J. Leibowitch was right: you can follow the 4/7 schedule, within the rules
- Eviplera ® (Complera ®) is eligible 4/7
- You can go direct to 4/7: bypassing 6/7, 5/7
- There was cheating in this trial
- There was sabotage in this unsponsored trial (see below)
- No intrinsic failure in this trial
- For patients 100% eligible and 100% adherent, regular VL inform us of nothing: they are useless
The subject is already controversial! And it is hilarious!
Prof. Philippe EVEN: Each clinical trial is a detective novel. It is primarily a novel because it is made-up, and it takes the spirit of a detective to detect all maneuvers.
At the min. 53:30 Video by Even.
Jacques Leibowitch fulminates!
The ANRS-4D trial tests, again, almost identically, what was tested with the Garches trials (ICCARRE-1 and-2). Minor difference: Eviplera® (Complera ®) is eligible (see description). And as a trial is a test of hypothesis, the hypothesis tested is: Had Leibowitch lied?
If a clinical trial is reran without its principal investigator, this trial is obviously meant to ensure that he did not lie. If we had trusted the team at Garches (Jacques Leibowitch Dominique Mathez, Truchis Stone, Damien Ledu, Jean Claude Melchior, Christian Perronne, 6 APHP [Paris Hospital Group] employees ...) we would have taken for granted their results: no failures with 94 patients. We find the same thing: this provides the evidence that Leibowitch et al. did not lie ... See the game here?... You, you did not know if the 6 APHP doctors had lied (tested hypothesis), but they, they knew the truth: must be hard to be put to the test in such fashion...
The results had been published under Pr. Perronne supervision. The re-trial insinuates that they had cheated... 10 authors had supposedly lied, so the trial is remade ...
And ANRS to say the result is encouraging (sic!). What the Fuck! And to announce 96% success. Not 100% ... 96 ... So this should be understood: they still insinuate that Leibowitch had lied by close to 4%.
We will drag these 4 pseudo-failures as a liability, because now, we have to repeat, again, on a larger scale, to be (finally ...) safe, safe, safe ... We will, from there on, keep an eye on the hypothesis tested in the Quartuor trial (yes, we will be following that too ...)
A typical trial report includes at least two types of failures: Intent to treat failures (ITT), and intrinsic failures. The ITT failure include everything: He leaves the country: failure; She suffers anxiety and exits the trial: failure; He commits suicide: failure. She interrupts treatment (toxicity): failure. The intrinsic failures, on the other hand, are limited to only failures reasonably attributable to the strategy: leaving for personal reasons is excluded, and when we have proof, we exclude cheaters!
Leibowitch, who had not cheated, will sniff cheating in ANRS-162-4D. He hassles de Truchis, who exposes the cheats: he has been wronged by 4 stowaways.
And ANRS falls into the trap.
Either they are morrons or bad guys ... or both
He started the trial and then panicks: Congratulations to recruiters!
Less than four weeks after the start of the trial: he feels bad, panicks and leaves the trial.
We cannot congratulate the recruiters!
Good that he is no cosmonaut ... On a journey to Mars ... De Truchis is no NASA!!!
This is upsetting ... This is a trial that opens the door to treatment for millions of people, in the waiting line because of shortages! This is more important than a trip to the moon!
Obviously, a good patient support will help! And now that we know the trial results, one can judge the responsibility of fearmongers who spread fear among patients!
This reminds us of the importance of freedom of choice. In the practical guide we had included a stepwise transition 6/7, 5/7: this may be irrelevant. Note however that the smooth transition is favorable to anxious patients.
Sir, you played us a bad trick here! Not only have you taken the place of motivated patients who have not been recruited for lack of spots, but we are now forced to repeat the trial because your defection hangs over the image of the protocol, that millions of patients are waiting for!
Discontinuation (for now): it does not count!
2 cheaters: revealed by their concentrations!
Confirmation dans cette video
No doping! Not cheating! No cheaters: dosage reveals who pees blue, and also those who do not take their meds at all!
Le Figaro exposes the cheating:
"Of the 4 patients failing, one quickly abandoned by fear, and two are unlikely to have followed their treatment," says Pierre de Truchis (Hospital Raymond Poincaré), who led the study.
Yes, of Truchis has indeed granted an interview! He leaches out here:
Dr. De Truchis: ... two of the three had low plasma levels of drugs which suggests they did not take quite the expected dose of drugs.
Who are the 2 patients in question? identifying 2 patients 3 on low dosage criterion is easy ... Since 1 of 3 patients always a largely measurable concentration.
Patient number 2 obviously take Atripla ®; he has Efavirenz concentrations, while ON, well above the average (3669 for an average of 2218 among 100 patients) and, while in OFF period, has 1543 (an average of 692 when OFF), which is even a concentration worthy of ON. If de Truchis accounts that patient #2 as one who does not take these meds, he will have clarify. Because, in the published poster, patient # 2 taking his medication properly.
This is confirmed here: https://www.youtube.com/watch?v=7RQgyObm0qQ
Who are those, then, who do not take the dose as ordered by the trial? Apart from # 1 and # 3? Whose doses are so low that they are not detected. Including in the period in which they were supposed to take meds. So that's quite clear.
You have undetectable concentrations when you are supposed to take medication and that you claim 100% adherence: you are lying!
Dear: Your doctor has prescribed Kivexa + 1 protease inhibitor, to be taken daily, and you miss doses? We understand you ... You are in a vicious circle: you are under PIs, you miss doses, your doctor realizes it, fears resistance, so leaves you under PIs. We understand your interest for the short cycle. We understand ... But you lied to the investigator ... Your lie costs us: redo the trial, delay deployment of the strategy for yet another 3, 4 or 5 years. The millions of patients who are waiting for a reliable strategy or simply treatment (because of shortages), do not say thank you! Here your lies have been damaging and are reprehensible.
Note for future trials: people want to go participate in a trial just to have an excuse to stop their treatment should not be included. This should be the first criteria for non-inclusion in the trial. Exclude anyone who would want to participate just to stop treatment.
We always thank the volunteers ... but not the liars!
Cheating (provisional): it does not count!
What is this illegitimate patient doing here? He was let in by a saboteur ...
And Figaro concludes: That leaves only one patient where the strategy failed to maintain undetectable viral load.
Really ??? Fortunately we understand that Le Figaro is linked to BigPharma (otherwise, how they would have known what de Truchis would / had leaked in Durban ... They were there?). Indeed, it is in the interest of BigPharma to suggest that there are intrinsic failures, just to maintain fear!
Le Figaro trips over the carpet. Let's read: That leaves only one patient where the strategy failed to maintain undetectable viral load.
The only one left is the patient # 2, under Atripla. We have met with him earlier: he takes his medication, he is not a cheater! Look well ... Is there not something that raises your eyebrow? Look closely ... It is not that obvious, but once you have seen it, you can never miss it.
Here is one clue. Let us read the Figaro (a scientific journal, as we all know): That leaves only one patient where the strategy failed to maintain undetectable viral load.
But ... in order maintain undetectability, the patient should at least be undetectable, in the first place...
Patient # 2 has a quantifiable viral load : it is quantified at 40! (not a printing error ...)
You can not maintain what you do not have. But who is stupid enough to let a patient whose viral load is low but detectable in a Short Cycle protocol; especially so close to the breaker: 50
Either they are morons or they are bad guys ... or both
Speaking of ANRS, it looks like we will have to settle for the third choice.
When specifying < 50, everyone understands: undetectectable with a detection/quantication thresold of 50. No one, in her right mind would include a detectable patient in such a protocole.
Jacques Leibowitch claims that "Short cycle shall be doctor supervised or won't be". Well, with such idiots in charge, it will most likely not be!...
Sir: You are a pawn in a game that is beyond you and a victim of over-medication, the wrong-medication. Atripla ® was, initially, 7315 mg / week and your VL remains detectable (40). You want Short Cycle, and, at 4180 mg / week, your VL 'explodes' to ... 55: so they will almost double dose from 4000 mg to 7000 mg. Your VL goes down! ... to 47 ... 3000 mg / week for 8 points of VL: 500 mg / week / point: You still have forty points to go ... Action! Let's increase the dose! A strategy doing badly? They double down. At this rate your virologist will join the government of France (or that of a pharmaceutical group). You are on the wrong foot: keep going!
Inclusion mistake : it does not count!
Number of intrinsic failures: ZERO
We would like to congratulate! but who? The careless ANRS recruiters
Who out did de Truchis? Just look at the recruiting centers. Well, ask de Truchis to publish recruitment centers we will see where from the two cheaters and their two accomplices are. Publish to avoid suspicion. Because, at this moment, the presence of the Parisian Virology Clique (Bichat, obviously ...) is highly suspicious... He may well not publish and keep the names under the carpet: which might be usefull to him.
Number of intrinsic failures: ZERO
Number of intrinsic failures: ZERO
Number of intrinsic failures: ZERO
This is the first study to answer beyond doubt on the question of intrinsic failures. Now that we have some answers, you can read previous studies with the understanding that there was no intrinsic failure, so they were extrinsic ... ANRS-4D revolutionizes short cycle outstanding issues were answered; we have to correct the practical guide accordingly.
Other things to look at
To avoid repetition, comments are added to each section of the poster (see below).
In short:
- No change in the proviral DNA (a poor measure of the reservoir): Not surprisingly. The 'reservoir' was on average rather high, often above the stupid Rouzioux criteria
- The liver likes it
- Cholesterol: we do not care; Cholesterol is innocent
- The pharmaceutical remission gain (and costs) are not explicitly detailed: it is simple: about 1/2 year per year per patient (which therefore gives them about 1 year of remission at the end of 2016)
Durban 2016: poster presented by P. de Truchis
Poster THPEB063: P. de Truchis (1), L. Assoumou (2), R. Landman (3), D. Mathez (4), J. Bellet (2), K. Amat (3), C. Katlama (5), P.M. Girard (6), D. Le Du (7), J. Izopet (8), B. Autran (9), M. Duracinski (10), J.C. Alvarez (11), D. Costagliola (2), C. Perronne (12) (1): APHP Hopital Raymond Poincare, CHU Paris Ile de France Ouest, Infectious Diseases, Garches, France, (2): Institut Pierre Louis Epidemiologie et Sante publique, INSERM, UPMC Universite
Paris 6, Paris, France, (3) IMEA, APHP CHU Bichat, Paris, France, (4) APHP CHU Paris Ile de France Ouest, Virology, Garches, France, 5APHP Hopital Pitie-Salpetriere, Universite Paris 06, Infectious Diseases, Paris, France, (6): APHP Hopital St Antoine, Universite Paris 06, Paris, France, (7) APHP Hopital R Poincare, Garches, France, 8CHU Toulouse, Hopital Purpan, Virology, Toulouse, France, (9) APHP Hopital Pitie-Salpetriere, Universite Paris 06, Immunology, Paris, France, (10) APHP CHU Bicetre, Paris, France, 11APHP Hopital R Poincare, Universite Versailles St Quentin, INSERM U1173, Pharmacology, Garches, France, (12) APHP Hopital R Poincare, Universite Versailles St Quentin, INSERM U1173, Infectious Diseases, Garches, France

Abstract
Background: Previous studies (FOTO, BREATHER) have given encouraging results with a 5/7 days Efavirenz-based maintenance regimen. Based on pilot experience (Leibowitch, FASEB .J. 2015), we conducted 48-week multicenter, open-label, single-arm prospective study evaluating efficacy and safety of 4/7 days maintenance therapy in HIV infected patients with controlled VL.
Methods: The main inclusion criteria were age > 18years; current regimen with 2 nucleoside analogs and either a boosted protease inhibitor PI/r or a NNRTI; no treatment modification in the last 6 months; VL < 50c/ml for at least one year; no resistance mutation to the drugs in the current regimen.
Maintenance therapy used the same regimen, taken 4 consecutive days of each week. Virological failure (VF) was defined as VL > 50c/ml confirmed within 4 weeks between D0-W48. Patients were evaluated at D0, W4, W8, W12, W16, W24, W32, W40, and W48. The study was designed to show that the efficacy of the strategy is superior to 80%, assuming a success rate equal to or above 90%, with a power of 87% and a 5% type-one error.
Values are presented as median [range]. Adherence to therapy was assessed by questionnaires, pill count, and MEMS caps for subgroup of patients.
Results: One hundred patients were included in the study, 82 men and 18 women, median age 47 [25-75], CD4 nadir 282 [7-1044] cells/µl, and receiving ARV therapy since 5.1 [1.3-25.2] years with VL < 50 since 4.1 [0.5-15.5] years. Current regimen included Tenofovir: TDF+FTC (89 patients) or abacavir+3TC (11 patients), combined with a PI/r for 29 individuals (Lopinavir/r:1, Atazanavir/r:13, Darunavir/r:15) or a NNRTI for 71 (EFV: 41, RPV: 25, ETV: 5). After 48 weeks, 96% [95% CI 90-98, Kaplan-Meier estimate] were still under maintenance 4/7 days regimen without failure; 1 patient returned to 7/7 regimen and left the study at W4, VF was confirmed in 3/100 patients at W4, W8, W40, with VL785, 124, and 969c/ml respectively. These 3 patients returned to 7/7 regimen and VL was subsequently suppressed in all 3.
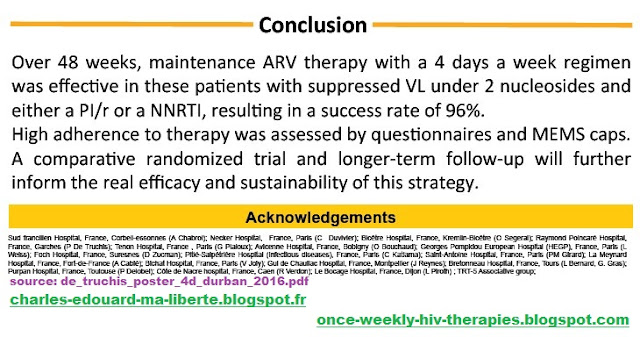
Conclusion: Over 48 weeks, maintenance ARV therapy with a 4days a week regimen was effective in these patients with suppressed VL under 2 nucleosides and either PI/r or NNRTI, resulting in success rate of 96%.

Background
Given the earlier recommended initiation of ART and the need for long life therapy, strategies reducing ART in take will have to be investigated for minimizing long-term cumulative toxicity of ARV drugs. A short cycle therapy strategy with planned short breaks from ART could be an alternative for reducing long-term toxic effects and costs. Previous studies (FOTO, BREATHER) have given encouraging results with a 5/7 days Efavirenz-based maintenance regimen.
Based on pilot experience (Leibowitch, FASEBJ-2015), we conducted a 48-week multicenter, open-label, single-arm prospective study evaluating efficacy and safety of a 4/7 days maintenance therapy in HIV infected patients with controlled VL.
Objective
The ANRS-162-4D study, short-cycle therapy strategy with 4-day on consecutively and 3-day off, aimed to evaluate the capacity of this strategy to maintain therapeutic success over 48 weeks in HIV-1-infected patients with controlled viral load for at least 12 months under antiretroviral treatment. Therapeutic success is defined by the absence of virologic failure (occurrence of two successive values of viral load > 50 copies/mL within 2 to 4 weeks apart), and the absence of study strategy discontinuation for more than 30 days.

Main inclusion criteria: age > 18years; current regimen with 2 nucleoside analogs and either a boosted protease inhibitor PI/r or NNRTI; no treatment modification in the last 4 months; plasma VL < 50c/ml for a least one year; no resistance mutation to the drugs in current regimen.
Patients were evaluated at D0, W4, W8, W12, W16, W24, W32, W40, W48 and W51.

Methods:
Primary endpoint: occurrence of therapeutic failure, as defined by 2 consecutive plasma viral load measurements > 50 copies/mL within two to four weeks, during the 48 weeks of follow-up (Virological failure) or discontinuation of the study/study strategy for more than 30 days (Strategic failure). ;
Secondary endpoints: tolerability, drug concentration, changes in CD4, CD4/CD8 ratio, metabolic parameters, and HIV DNA at week 48. For these secondary endpoints missing data were replaced by the last observation. Genotypic resistance test performed in patients in case of virological failure. ;
Adherence to the study strategy: assessed by self-reported questionnaires, pill count, drug concentrations, and MEMS caps for sub group of patients.
; Quality of life and felt symptoms: assessed by self-reported questionnaires a week 0, 24 and 48.
The study was designed to show that the efficacy of the strategy is superior to 80%, assuming a success rate equal to or above 90%, with power of 87% and 5% one-type error.
A maximum of 10 treatment failures including a maximum of 5 virological failures was expected over the study for success. The viral load monitoring was on line and the DSMB decision was required every two virological failures.

Study flowchart

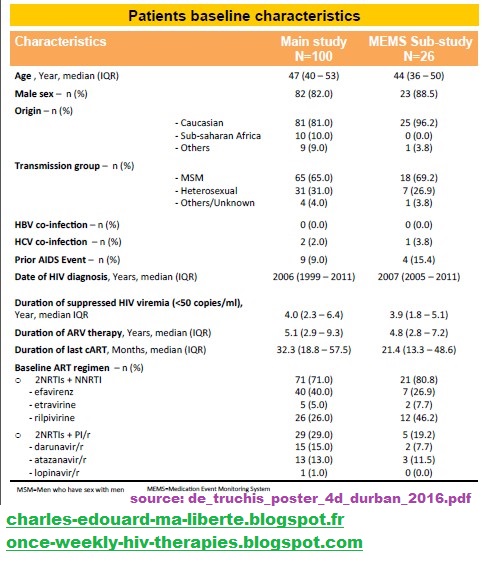
| Patients baseline characteristics | |||
| Characteristics |
Main study N=100 | MEMS Sub-study N=26 | |
| Age, Year, median (IQR) | 47 (40-53) | 44 (36-50) | |
| Male sex–n (%) | 82 (82.0) | 23 (88.5) | |
| Origin(%) | |||
| Caucasian | 81 (81.0) | 25 (96.2) | |
| Sub-saharan Africa | 10 (10.0) | 0 (0) | |
| Others | 9 (9.0) | 1 (3.8) | |
| Transmission group–n (%) | |||
| MSM | 65(65.0) | 25 (96.2) | |
| Heterosexual | 31 (31.0) | 7 (26.9) | |
| Others/Unknown | 9 (9.0) | 1 (3.8) | |
| HBV co-infection(%) | 0 (0.0) | 0 (0.0) | |
| HCV co-infection(%) | 2 (2.0) | 1 (3.8) | |
| Prior AIDS Event–n(%) | 9 (9.0) | 4 (15.4) | |
| Date of HIV diagnosis Years median (IQR) | 2006 (1999-2011) | 2007 (2005-2011) | |
| Duration of suppressed HIV viremia(< 50copies/ml), Year, median IQR | 4.0 (2.3 - 6.4) | 3.9(1.8 - 5.1) | |
| Duration of ARV therapy Years, median(IQR) | 5.1 (2.9-9.3) | 4.8 (2.8-7.2) | |
| Duration of last cART Months, median (IQR) | 32.3 (18.8-57.5) | 21.4 (13.3-48.6) | |
| Baseline ART regimen n (%) | |||
| o | 2NRTIs NNRTI | 71 (71.0) | 21 (80.8) |
| - Efavirenz | 40 (40.0) | 7 (26.7) | |
| - Etravirine | 5 (5.0) | 2 (7.7) | |
| - Rilpivirine | 26 (26.0) | 12 (46.2) | |
| o | 2NRTIsPI/r | 29 (29.0) | 5 (19.2) |
| - Darunavir/r | 15 (15.0) | 2 (7.7) | |
| - Atazanavir/r | 13 (13.0) | 3 (11.5) | |
| - Lopinavir/r | 1 (1.0) | 0 (0.0) | |
| MSM = Men who have sex with men; MEMS = Medication Event Monitoring System | |||
Conclusion: maintenance ARV therapy with a 4 days a week regimen was effective in these patients with suppressed VL under 2 either PI/r or NNRTI, resulting in success rate of 96%. High adherence to therapy was assessed by questionnaires and MEMS caps. A comparative randomized trial and longer‐term follow‐up will further inform the real efficacy and sustainability of this strategy.

Patients with therapeutic failure N °c ART Age Sex CDC Duration of VL suppression (Year) pVL pre cART(cp/ml) CD4 Nadir (/mm3) CD4 (/mm3)pVL (cp/ml)ART concentration (ng/ml) Resistance Self reported Adherence DO W48 D0 failure W48 1st VL rebound VL Control 1 ABC 48 M C 6.7206811251119814<4027(S04) <40OFF3TC CTR (13 LPV:< 2 LPV/r days after): 785 2 TDF 5 2 M A 5.9310002095745474012(S12) 47 OFF FTC CTR ( 9days EFV: 1543 EFVa per): 55 study. OFF No 100% LPV:< 75RTV:< 10 ON No 100% TDF:71 FTC:207 EFV:3669 3 ABC 31 F A 4.2650042211361229< 2096(S40) <20 ON ON No 100% 3TC CTR (22 ATV:< 20 ATV: 4190ATV/r days aper):RTV: 1080227 4 TDF 35 M A 3.01498330500<20<20 OFF Discontinuation by ptat100% FTC(S04)EFV:709W04,related to the EFV study strategy





Date: July 19, 2016
Source: ANRS
Summary: Triple antiretroviral therapy taken just 4 days a week, instead of daily, kept plasma viral load below 50 copies/mL in 96 of 100 patients in the study ANRS 162-4D.
Simplification of treatment use is designed to lower rates of side effects and treatment costs, and to improve acceptability and adherence. It is important for people living with HIV, whose antiretroviral therapy is lifelong. This is why it is being studied in several trials worldwide. ANRS is assessing the value of reducing drug dosages or their frequency, and the value of sparing treatment options. In France, the ANRS 165 DARULIGHT trial is evaluating the benefits of halving the daily dose Darunavir ; ANRS 167 LAMIDOL is assessing combination therapy with Lamivudine and Dolutegravir, and ANRS 163 ETRAL is evaluating another combination therapy, Etravirine-Raltegravir. The ANRS 162-4D trial is studying the effect of limited frequency of antiretrovirals intake. Dr Pierre de Truchis (Hôpital Raymond Poincaré, Garches, France) presents the results of ANRS 162-4D in a poster at AIDS 2016 in Durban, South Africa (18 to 22 July).
ANRS 162-4D
The ICCARRE project headed by Professor Jacques Leibowitch (Infectious Diseases Department, Hôpital Raymond Poincaré, Garches, France) yielded encouraging results in patients whose treatment was reduced to 5 and then to 4 days a week, or even less for some patients (FASEB Journal, 2015).
To confirm these observations, in 2014 the ANRS started a prospective, multicenter, nonrandomized trial (ANRS 162-4D) run by Professor Christian Perronne (Hôpital Raymond Poincaré, Garches, France) in which patients received the same antiretroviral treatment regimen over 48 weeks. The aim was to assess whether treatment taken on 4 consecutive days a week by HIV-positive patients would keep plasma viral load below 50 copies/mL. The 100 patients had been taking triple antiretroviral therapy for an average of 5 years and their viral load had been undetectable for 4 years. Their combination therapy comprised 2 nucleoside analogues plus a non-nucleoside reverse transcriptase inhibitor or a protease inhibitor.
The results presented in Durban are encouraging. After 48 weeks, 96% of patients were still on the 4 days a week regimen and had a viral load below 50 copies/mL. Only three patients had a detectable viral load at weeks 4, 12, and 40 (respectively, 785, 124, and 969 copies/mL). In these patients, viral load dropped below the detection threshold upon return to the 7 days a week treatment regimen, without appearance of resistance. One patient dropped out of the study at week 4.
These data were completed by a concomitant analysis of treatment adherence in a subgroup of patients using self-report questionnaires, assays of blood drug levels, and counting of drug doses taken using electronic pillboxes. Dr Pierre de Truchis noted that "The analysis of treatment adherence showed that the 4 times a week regimen was well adhered to and accepted by the patients. In over 90% of cases, drug intake matched the prescription."
This innovative strategy now needs to be confirmed in a randomized trial comparing two groups of patients. This is the aim of ANRS QUATUOR, a trial which will be conducted in more patients over a longer period using more recent antiretrovirals such as integrase inhibitors, which are now the mainstay of treatment. Of 640 patients recruited in several hospital centers, half will receive treatment 4 days a week and the other half 7 days a week, for 48 weeks. If similar results are noted in the two groups, all patients will be put on the 4 times a week regimen for a further 48 weeks. The aim is to show that the 4 times a week regimen is not inferior to the everyday regimen, ie, the efficacy is the same and the patients on treatment 4 days a week experience additional benefits (fewer side effects, better adherence...).
Professor Jean-François Delfraissy, Director of ANRS, observed that "These results encourage us to pursue our aims of improving quality of life on treatment and meeting a strong demand from some patients for a lower drug burden." Should the 4 days a week regimen now be recommended in everyday practice? "Only a randomized trial will be able to approve this strategy," says Professor Delfraissy. Current international recommendations stipulate that continuous treatment should be initiated as soon as possible after discovering positive HIV serostatus, regardless of CD4 cell count.





No comments:
Post a Comment
Note: Only a member of this blog may post a comment.